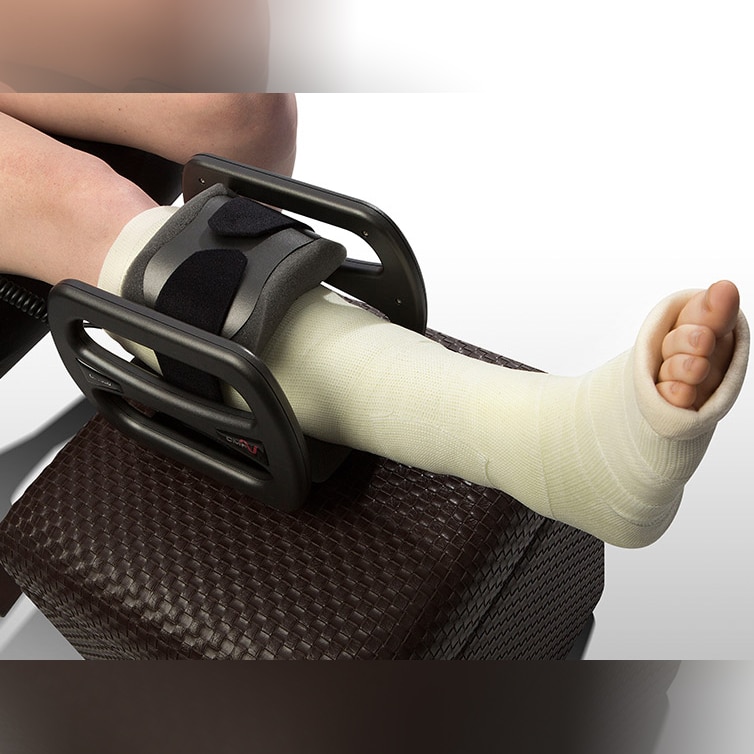
CMF OL1000 Bone Growth Stimulators
$3,500.00
CMF OL1000 Bone Growth Stimulators are portable, battery-powered medical devices indicated for use in the noninvasive treatment of an established nonunion fracture acquired secondary to trauma, excluding all vertebrae and flat bones. The device has the following features:
- Lightweight and comfortable
- Easy-to-use & Noninvasive
- Requires simple, one-button operation
- Device is worn for 30 minutes per day
- Can be used with internal or external fixation or over a cast
PRESCRIPTION REQUIRED
Notice: Listed prices are for out-of-pocket purchases only. When using insurance, claims must be billed at the insurance company’s set fee schedule rates, which may differ from our out-of-pocket price.
💵
Exclusive Online Price
🚚
Get Free Shipping on All Orders Over $100
💳
CARE CREDIT IS AVAILABLE
CMF OL1000 Bone Growth Stimulators are Portable | Michigan USA
CMF OL1000 Bone Growth Stimulators
 The CMF OL1000 Bone Growth Stimulators are portable, battery powered medical devices indicated for use in the noninvasive treatment of an established nonunion fracture acquired secondary to trauma, excluding all vertebrae and flat bones.
The CMF OL1000 Bone Growth Stimulators are portable, battery powered medical devices indicated for use in the noninvasive treatment of an established nonunion fracture acquired secondary to trauma, excluding all vertebrae and flat bones.
The CMF OL1000 device has the following features:
- Lightweight and comfortable Bone Growth Stimulators
- Easy to use & Noninvasive
- Requires simple, one button operation CMF OL1000
- Device is worn for only 30 minutes per day
- Can be used with internal or external fixation or over a cast
This CMF OL1000 treatment has been shown in pre-clinical studies to help the body’s own healing process begin working. Clinical studies have shown. CMF OL1000 an increased chance of healing of 60.7% in patients with nonunions that averaged 29.3 months from injury. Registry data on over 2300 patients reports a heal rate of 75.1%.
CONTRAINDICATIONS CMF OL1000 Bone Growth Stimulators
The Use of this CMF OL1000 device is contraindicated in individuals having a synovial pseudarthrosis. Physicians should not prescribe CMF 0L1000 for applications that may place the treatment transducers in close proximity to the pacemaker.
WARNINGS;
The CMF OL1000 safety and effectiveness of the use of this CMF OL1000 device. on individuals lacking skeletal maturity have not been established. Animal studies conducted to date do not suggest any long-term significant adverse effects from use of this device. However., CMF OL1000 long-term effects in humans are unknown. The safety of use of this device during pregnancy or nursing in humans has not been established.
PRECAUTIONS
The Bone Growth Stimulators safety and effectiveness of the use of this CMF OL1000 device on individuals with nonunion secondary to, or in conjunction with. a pathological condition have not been established.
This CMF OL1000 device
should not be used CMF OL1000 if there are mental or physical conditions. that preclude patient compliance with the physician and device instructions. CMF OL1000 When conditions of atrophy are present or when fractures have remained unhealed for long periods of time; there may be less successful results.
ADVERSE EFFECTS CMF OL1000
No known significant adverse effects have resulted from the use of this Bone Growth Stimulators CMF OL1000 device. Clinical studies, animal studies.
and tissue culture experiments conducted with the OL1000., which has the same treatment signal as the CMF OL1000 SC1 have not indicated any evidence of significant adverse effects.
This document addresses the use noninvasive electrical CMF OL1000 bone growth stimulation devices for the treatment of orthopedic and neurosurgical conditions.
of the appendicular skeleton. This document does not address invasive electrical bone growth stimulation of any area of the body or noninvasive electrical CMF OL1000 bone growth stimulation of the spine.
Note:
Please refer to the following document for additional information related to devices used to stimulate bone growth
Medically Necessary:
At least 45 days have passed since the date of fracture or the date of surgical treatment of the fracture and
Serial radiographs or appropriate imaging studies confirm that no progressive signs of healing have occurred and
The fracture gap is less than 1 centimeter.
Not Medically Necessary
Draining osteomyelitis or
Synovial pseudoarthroses.
As an adjunct to that is at the time of or immediately after bunionectomy procedures Note; When such surgery results in nonunion the medically necessary criteria above may apply or
As an adjunct to that is at the time of or immediately after distraction osteogenesis procedures for any indication for example limb lengthening nonunion or tibial defects or
Delayed/incomplete union fractures or
Fresh fractures or
Patellar tendinopathy or
Pathological fractures due to bone pathology or tumor/malignancy or
Stress fractures
CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000
| Weight | 5 lbs |
|---|---|
| Dimensions | 12 × 12 × 12 in |
Related products
-


CMF SPINALOGIC
$2,950.00
CMF OL1000 Bone Growth Stimulators
$3,500.00
CMF OL1000 Bone Growth Stimulators are portable, battery-powered medical devices indicated for use in the noninvasive treatment of an established nonunion fracture acquired secondary to trauma, excluding all vertebrae and flat bones. The device has the following features:
- Lightweight and comfortable
- Easy-to-use & Noninvasive
- Requires simple, one-button operation
- Device is worn for 30 minutes per day
- Can be used with internal or external fixation or over a cast
PRESCRIPTION REQUIRED
Notice: Listed prices are for out-of-pocket purchases only. When using insurance, claims must be billed at the insurance company’s set fee schedule rates, which may differ from our out-of-pocket price.
💵
Exclusive Online Price
🚚
Get Free Shipping on All Orders Over $100
💳
CARE CREDIT IS AVAILABLE
CMF OL1000 Bone Growth Stimulators are Portable | Michigan USA
CMF OL1000 Bone Growth Stimulators
 The CMF OL1000 Bone Growth Stimulators are portable, battery powered medical devices indicated for use in the noninvasive treatment of an established nonunion fracture acquired secondary to trauma, excluding all vertebrae and flat bones.
The CMF OL1000 Bone Growth Stimulators are portable, battery powered medical devices indicated for use in the noninvasive treatment of an established nonunion fracture acquired secondary to trauma, excluding all vertebrae and flat bones.
The CMF OL1000 device has the following features:
- Lightweight and comfortable Bone Growth Stimulators
- Easy to use & Noninvasive
- Requires simple, one button operation CMF OL1000
- Device is worn for only 30 minutes per day
- Can be used with internal or external fixation or over a cast
This CMF OL1000 treatment has been shown in pre-clinical studies to help the body’s own healing process begin working. Clinical studies have shown. CMF OL1000 an increased chance of healing of 60.7% in patients with nonunions that averaged 29.3 months from injury. Registry data on over 2300 patients reports a heal rate of 75.1%.
CONTRAINDICATIONS CMF OL1000 Bone Growth Stimulators
The Use of this CMF OL1000 device is contraindicated in individuals having a synovial pseudarthrosis. Physicians should not prescribe CMF 0L1000 for applications that may place the treatment transducers in close proximity to the pacemaker.
WARNINGS;
The CMF OL1000 safety and effectiveness of the use of this CMF OL1000 device. on individuals lacking skeletal maturity have not been established. Animal studies conducted to date do not suggest any long-term significant adverse effects from use of this device. However., CMF OL1000 long-term effects in humans are unknown. The safety of use of this device during pregnancy or nursing in humans has not been established.
PRECAUTIONS
The Bone Growth Stimulators safety and effectiveness of the use of this CMF OL1000 device on individuals with nonunion secondary to, or in conjunction with. a pathological condition have not been established.
This CMF OL1000 device
should not be used CMF OL1000 if there are mental or physical conditions. that preclude patient compliance with the physician and device instructions. CMF OL1000 When conditions of atrophy are present or when fractures have remained unhealed for long periods of time; there may be less successful results.
ADVERSE EFFECTS CMF OL1000
No known significant adverse effects have resulted from the use of this Bone Growth Stimulators CMF OL1000 device. Clinical studies, animal studies.
and tissue culture experiments conducted with the OL1000., which has the same treatment signal as the CMF OL1000 SC1 have not indicated any evidence of significant adverse effects.
This document addresses the use noninvasive electrical CMF OL1000 bone growth stimulation devices for the treatment of orthopedic and neurosurgical conditions.
of the appendicular skeleton. This document does not address invasive electrical bone growth stimulation of any area of the body or noninvasive electrical CMF OL1000 bone growth stimulation of the spine.
Note:
Please refer to the following document for additional information related to devices used to stimulate bone growth
Medically Necessary:
At least 45 days have passed since the date of fracture or the date of surgical treatment of the fracture and
Serial radiographs or appropriate imaging studies confirm that no progressive signs of healing have occurred and
The fracture gap is less than 1 centimeter.
Not Medically Necessary
Draining osteomyelitis or
Synovial pseudoarthroses.
As an adjunct to that is at the time of or immediately after bunionectomy procedures Note; When such surgery results in nonunion the medically necessary criteria above may apply or
As an adjunct to that is at the time of or immediately after distraction osteogenesis procedures for any indication for example limb lengthening nonunion or tibial defects or
Delayed/incomplete union fractures or
Fresh fractures or
Patellar tendinopathy or
Pathological fractures due to bone pathology or tumor/malignancy or
Stress fractures
CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000
| Weight | 5 lbs |
|---|---|
| Dimensions | 12 × 12 × 12 in |
Related products
-


CMF SPINALOGIC
$2,950.00
CMF OL1000 Bone Growth Stimulators
$3,500.00
CMF OL1000 Bone Growth Stimulators are portable, battery-powered medical devices indicated for use in the noninvasive treatment of an established nonunion fracture acquired secondary to trauma, excluding all vertebrae and flat bones. The device has the following features:
- Lightweight and comfortable
- Easy-to-use & Noninvasive
- Requires simple, one-button operation
- Device is worn for 30 minutes per day
- Can be used with internal or external fixation or over a cast
PRESCRIPTION REQUIRED
Notice: Listed prices are for out-of-pocket purchases only. When using insurance, claims must be billed at the insurance company’s set fee schedule rates, which may differ from our out-of-pocket price.
💵
Exclusive Online Price
🚚
Get Free Shipping on All Orders Over $100
💳
CARE CREDIT IS AVAILABLE
CMF OL1000 Bone Growth Stimulators are Portable | Michigan USA
CMF OL1000 Bone Growth Stimulators
 The CMF OL1000 Bone Growth Stimulators are portable, battery powered medical devices indicated for use in the noninvasive treatment of an established nonunion fracture acquired secondary to trauma, excluding all vertebrae and flat bones.
The CMF OL1000 Bone Growth Stimulators are portable, battery powered medical devices indicated for use in the noninvasive treatment of an established nonunion fracture acquired secondary to trauma, excluding all vertebrae and flat bones.
The CMF OL1000 device has the following features:
- Lightweight and comfortable Bone Growth Stimulators
- Easy to use & Noninvasive
- Requires simple, one button operation CMF OL1000
- Device is worn for only 30 minutes per day
- Can be used with internal or external fixation or over a cast
This CMF OL1000 treatment has been shown in pre-clinical studies to help the body’s own healing process begin working. Clinical studies have shown. CMF OL1000 an increased chance of healing of 60.7% in patients with nonunions that averaged 29.3 months from injury. Registry data on over 2300 patients reports a heal rate of 75.1%.
CONTRAINDICATIONS CMF OL1000 Bone Growth Stimulators
The Use of this CMF OL1000 device is contraindicated in individuals having a synovial pseudarthrosis. Physicians should not prescribe CMF 0L1000 for applications that may place the treatment transducers in close proximity to the pacemaker.
WARNINGS;
The CMF OL1000 safety and effectiveness of the use of this CMF OL1000 device. on individuals lacking skeletal maturity have not been established. Animal studies conducted to date do not suggest any long-term significant adverse effects from use of this device. However., CMF OL1000 long-term effects in humans are unknown. The safety of use of this device during pregnancy or nursing in humans has not been established.
PRECAUTIONS
The Bone Growth Stimulators safety and effectiveness of the use of this CMF OL1000 device on individuals with nonunion secondary to, or in conjunction with. a pathological condition have not been established.
This CMF OL1000 device
should not be used CMF OL1000 if there are mental or physical conditions. that preclude patient compliance with the physician and device instructions. CMF OL1000 When conditions of atrophy are present or when fractures have remained unhealed for long periods of time; there may be less successful results.
ADVERSE EFFECTS CMF OL1000
No known significant adverse effects have resulted from the use of this Bone Growth Stimulators CMF OL1000 device. Clinical studies, animal studies.
and tissue culture experiments conducted with the OL1000., which has the same treatment signal as the CMF OL1000 SC1 have not indicated any evidence of significant adverse effects.
This document addresses the use noninvasive electrical CMF OL1000 bone growth stimulation devices for the treatment of orthopedic and neurosurgical conditions.
of the appendicular skeleton. This document does not address invasive electrical bone growth stimulation of any area of the body or noninvasive electrical CMF OL1000 bone growth stimulation of the spine.
Note:
Please refer to the following document for additional information related to devices used to stimulate bone growth
Medically Necessary:
At least 45 days have passed since the date of fracture or the date of surgical treatment of the fracture and
Serial radiographs or appropriate imaging studies confirm that no progressive signs of healing have occurred and
The fracture gap is less than 1 centimeter.
Not Medically Necessary
Draining osteomyelitis or
Synovial pseudoarthroses.
As an adjunct to that is at the time of or immediately after bunionectomy procedures Note; When such surgery results in nonunion the medically necessary criteria above may apply or
As an adjunct to that is at the time of or immediately after distraction osteogenesis procedures for any indication for example limb lengthening nonunion or tibial defects or
Delayed/incomplete union fractures or
Fresh fractures or
Patellar tendinopathy or
Pathological fractures due to bone pathology or tumor/malignancy or
Stress fractures
CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000 CMF OL1000
| Weight | 5 lbs |
|---|---|
| Dimensions | 12 × 12 × 12 in |
Related products
-


CMF SPINALOGIC
$2,950.00